RISK-HUNT3R is the new European effort hosting world-leading experts from many different disciplines aiming to develop a novel and modular framework for animal-free next generation risk assessment (NGRA).
Over the past decades, science and society have increasingly started demanding a paradigm shift towards chemical safety and risk assessment without using animals. In addition to growing ethical concerns around the use of animal studies, there is the fundamental need to address all potential health effects relevant and specific to humans. Furthermore, the limited efficiency of animal-based testing (low throughput, high costs, insufficient laboratory capacity) will not be able to keep up the pace in ensuring the required protection of European citizens based on the sheer number of chemicals that require safety assessment.
The vision of the RISK-HUNT3R consortium is to establish a reliable and cost-effective chemical risk assessment strategy based entirely on non-animal approaches.
Scientific advances in in silico and in vitro testing, via so-called new approach methodologies (NAMs), have greatly increased the opportunities for application of NAMs in chemical safety testing.
“Despite successful integration of various NAMs in pre-marketing chemical safety assessment, the remaining challenges for animal-free approaches in risk assessment have become more evident.” says Prof. Hennicke Kamp, Head of Services Experimental Toxicology and Ecology at BASF and member of the project’s Executive Office.
The RISK-HUNT3R vision is that the route towards the solution to NGRA-based testing can be achieved through the combination of human exposure scenarios, in vitro hazard assessment, and NAM-based toxicokinetic and toxicodynamic testing, and the integration of these in a risk assessment framework using computational approaches and final decisions based on weight of evidence.
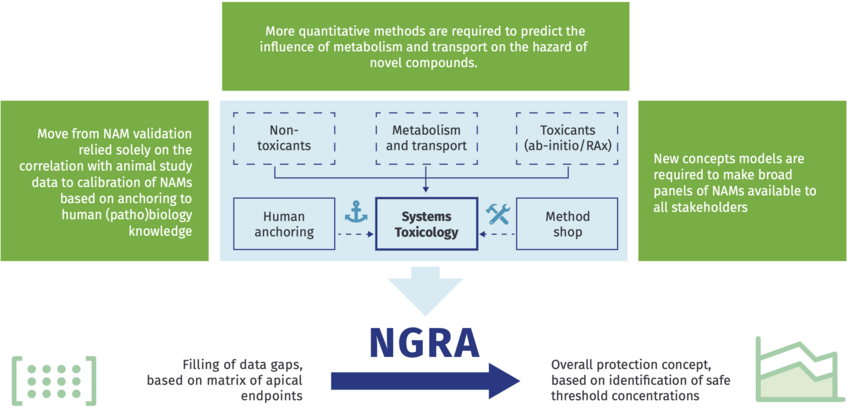
Source: RISK-HUNT3R Flyer
The project launches in June 2021 and will run for 5 years with a budget of €23M granted by Europe’s Horizon 2020 program. RISK-HUNT3R unites 37 partners, academic researchers, regulatory authorities, and safety scientists from key industry sectors, to prove that NGRA can be put into practice.
Differently from previous efforts, regulatory expertise has been part of the consortium since the proposal phase.
“This will allow translating the research effort into fit-for-purpose risk assessment frameworks and strategies in a straightforward manner”, emphasizes Dr. Mirjam Luijten, lead scientist at the National Institute for Public Health and the Environment (RIVM) and RISK-HUNT3R Executive Office member.
The project will develop, validate and implement integrated approaches to NGRA using innovative, mechanism-based, and human-relevant in silico and in vitro methodologies.
“We will bundle, under one roof, different innovative test systems and cutting- edge in silico models for hazard identification, and integrate these with systematic evaluation of human exposure scenarios” explains Prof. Marcel Leist, who is also in the Executive Office as well as chair of the in vitro Toxicology and Biomedicine Department at the University of Konstanz.
The trailblazing NAM-based NGRA strategy will be established with state-of-the-art technology and scientific knowledge, and will provide a framework for chemical safety assessment that is truly human-centric. Moreover, it will cater to regulatory needs whilst allowing for uptake of emerging innovations. Therefore, anticipation is that it will find wide application in multiple regulatory contexts, across industry sectors, and in protecting different population groups, throughout all life stages, including the most vulnerable, children, pregnant women, and elderly.
The aim of the RISK-HUNT3R project to better protect citizens against hazardous chemicals through non-animal methods while enabling the development of safe and sustainable alternatives is in line with the toxic-free environment goal of the European Commission’s Green Deal.
“The integrated testing strategy comprising in silico and in vitro test methods, will provide unprecedented and reliable protection of the human population against chemical-related health effects”, concludes Prof. Bob van de Water, head of the Division of Drug Discovery and Safety of the Leiden Academic Centre for Drug Research and coordinator of the project.
Factsheet
- RISK-HUNT3R stands for RISK assessment of chemicals integrating HUman centric Next generation Testing strategies promoting the 3Rs.
- The 3R principles are Replacement, Reduction and Refinement of animal-based models.
- The consortium counts on 37 partners (40% industry, 55% academia and 5% regulatory organizations) and is coordinated by the University of Leiden.
- Budget of €23 million, granted by the Horizon 2020 EC program.
- The project will be officially launched in June 2021 and be funded for 5 years.
- RISK-HUNT3R is one of three projects constituting the research cluster ASPIS (Animal-free Safety assessment of chemicals: Project cluster for Implementation of novel Strategies). Together with the projects ONTOX and PrecisionTOX, this will represent Europe’s €60M effort towards the sustainable, animal-free and reliable chemical risk assessment of tomorrow.

