- By clicking on the following link before January 31, 2020 you will be taken directly to the final version of the article on ScienceDirect, which you are welcome to read or download. No sign up, registration or fees are required: https://authors.elsevier.com/c/1aDQJ6T90imb8
Abstract
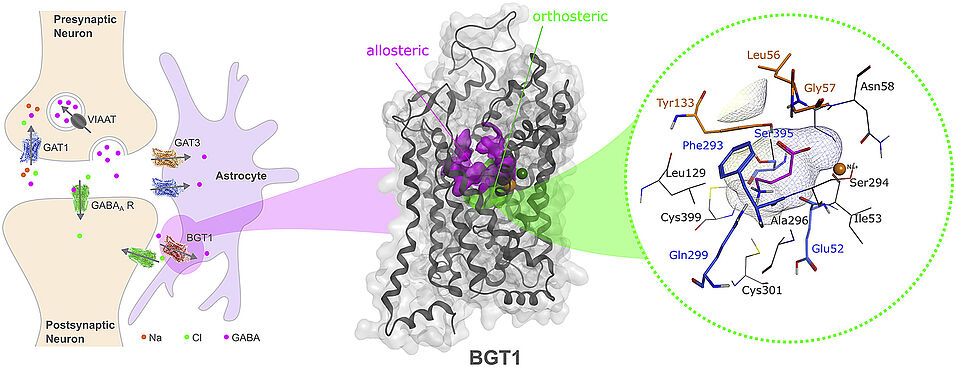
ɣ-aminobutyric-acid (GABA) functions as the principal inhibitory neurotransmitter in the central nervous system. Imbalances in GABAergic neurotransmission are involved in the pathophysiology of various neurological diseases such as epilepsy, Alzheimer's disease and stroke. GABA transporters (GATs) facilitate the termination of GABAergic signaling by transporting GABA together with sodium and chloride from the synaptic cleft into presynaptic neurons and surrounding glial cells. Four different GATs have been identified that all belong to the solute carrier 6 (SLC6) transporter family: GAT1-3 (SLC6A1, SLC6A13, SLC6A11) and betaine/GABA transporter 1 (BGT1, SLC6A12). BGT1 has emerged as an interesting target for treating epilepsy due to animal studies that reported anticonvulsant effects for the GAT1/BGT1 selective inhibitor EF1502 and the BGT1 selective inhibitor RPC-425. However, the precise involvement of BGT1 in epilepsy remains elusive because of its controversial expression levels in the brain and the lack of highly selective and potent tool compounds. This review gathers the current structural and functional knowledge on BGT1 with emphasis on brain relevance, discusses all available compounds, and tries to shed light on the molecular determinants driving BGT1 selectivity.
This article is part of the issue entitled ‘Special Issue on Neurotransmitter Transporters’.
Reprinted from Neuropharmacology ‘Special Issue on Neurotransmitter Transporters’, Volume 161, 15 December 2019, 107644, Stefanie Kickinger, Eva Hellsberg, Bente Frølund, Arne Schousboe, Gerhard F. Ecker and Petrine Wellendorph, Structural and molecular aspects of betaine-GABA transporter 1 (BGT1) and its relation to brain function, © 2019 Published by Elsevier Ltd., with permission from Elsevier.