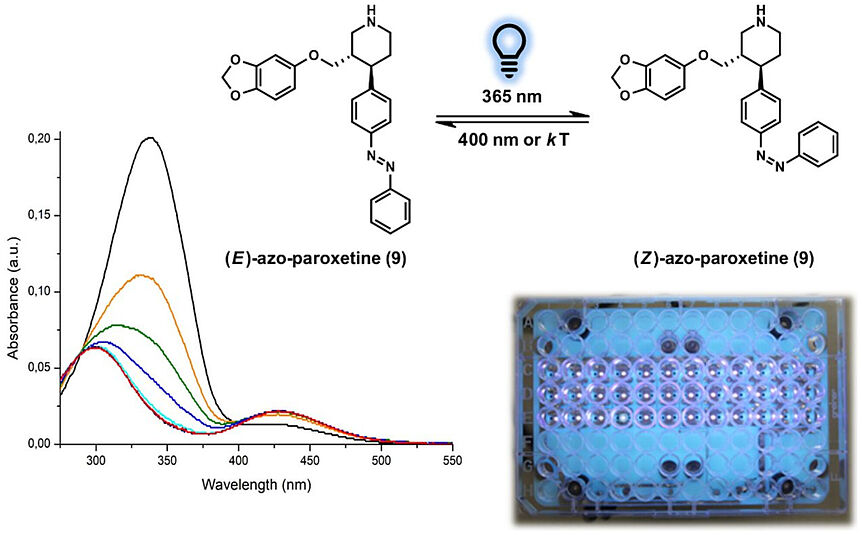
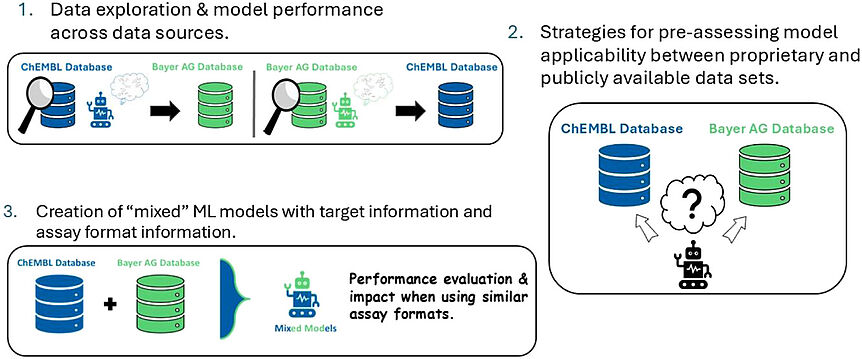
Abstract
The human serotonin and dopamine transporters (hSERT and hDAT) facilitate thefast and selective reuptake of their endogenous substrates, the neurotransmitters serotonin and dopamine, respectively. Both transporters belong to the solute carrier 6 protein family and share high sequence identity, the same protein fold and transport mechanism. Numerous compound classes have been identified to interact with these transporters, whichare used in therapeutic settings or abused as illicit drugs.
This thesis focuses on the molecular interaction profiles of hSERTand hDATby using different computational methods. The results and challenges in this field are presented in form of three independent, peer-reviewed studies. At first, the chemical compound space was explored by retrieving scaffold clusters and biological measurements from linked open data sources. A consistentdata setwas chosen from the results toinvestigate the selectivity trends in hSERT and hDATwith studies of structure-activity relationships, protein-ligand docking, and molecular dynamics simulations. Subsequently, the molecular determinants for selectivity in both transporters were studied in detail with exemplary representatives from the same compound class by biochemical, computational, and electrophysiological approaches. The computational part comprised characterization of the substituents with molecular descriptors, induced-fit docking, and a solvent analysis in the ligand binding pocket.
Finally, a serotonin-bound homology model of the human serotonin transporter was built in an outward-occluded conformation, a key intermediate in the physiological transport cycle, where the substrate interactions can be optimally studied. Molecular dynamics simulations of the outward-occluded and outward-open transporter conformations, important molecular features of these states werecompared by monitoring the extra-and intracellular gating residues, permeationpathway solvation, and the protein-ligand interactions in the central binding site.
Taken together, this work provides a useful contribution to enhance our understanding of molecular interactions regarding transporter selectivity and transport mechanism, but many questions remain elusive for upcoming future studies.
Eva was supported by the PhD program "MolTag".